VIRAL HEPATITIS
Hepatitis A
Hepatitis B
Cases = 101
Crude Rate (per 100,000 population) = 1.8
Age-adjusted race-specific rates (per 100,000 population)White = 0.9
Nonwhite = 3.1
Sex-specific rates
(per 100,000 population)Male = 2.5
Female = 1.1
Hepatitis B is a bloodborne pathogen disease transmitted by direct contact with blood or body fluids that contain the virus, sexual contact with an infected person, or from an infected mother to her infant during childbirth. In approximately 20% of reported individuals, risk factors for infection are not known.
A safe effective vaccine is available for hepatitis B and is recommended for routine administration to infants as part of the routine immunization series. Catch-up immunization is also recommended for adolescents.
All pregnant women should be screened for HBsAG at the earliest prenatal visit. Infants born to HBsAG(+) mothers should receive hepatitis B immune globulin (HBIG) within 12 hours of birth in addition to hepatitis B vaccine.
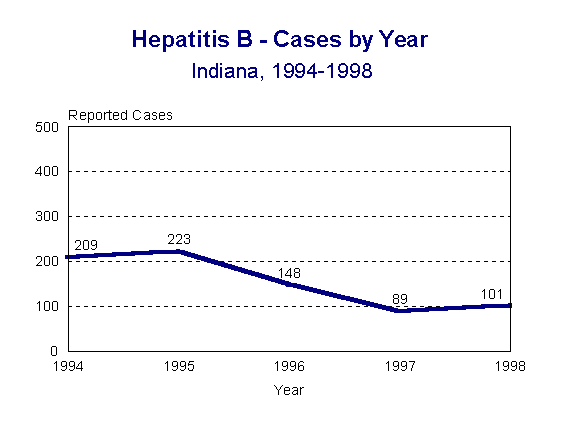
In 1998, there was a slight increase in reported cases of acute hepatitis B. Figure 1 shows the number of cases by year since 1994.
Figure 1.
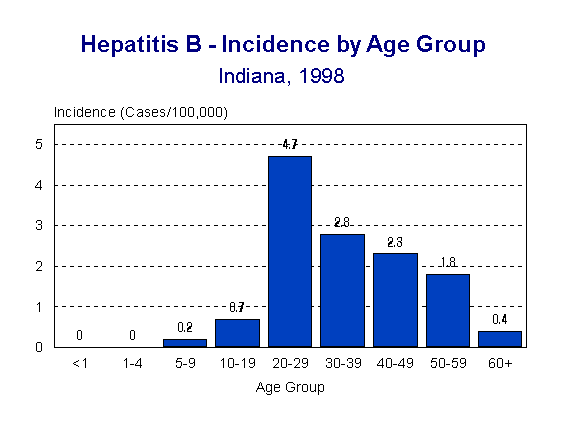
The rate of acute hepatitis B infection varied with age. The highest rate in Indiana is found in young adults, aged 20-29 (4.7/100,000 population). Figure 2 shows the rate of acute hepatitis B by age group.
Figure 2.
Nonwhites (3.1/100,000) were over three times more likely to become infected than whites (0.9). Eighty percent of the cases reported jaundice, and 20% were hospitalized for hepatitis.
Risk factors for contracting hepatitis B were known for 84 cases (84%) and are shown in Table 1. There were two medical employees reported with acute hepatitis B. Both had been received hepatitis B vaccine but did not have a hepatitis B antibody titer after vaccination. One case reported the only risk factor as a tattoo application four months prior to the hepatitis B infection. One person, with no additional risk factors, became infected with hepatitis B after receiving a blood transfusion and died.
Table 1.
Frequency of Patient-Recorded Risk Factors for Hepatitis B
Indiana, 1998
Risk Factor |
Number of "Yes" responses* |
| Homosexual/bisexual | 21 |
| Multiple sexual partners | 18 |
| History of dental work | 10 |
| Injectable drug use | 8 |
| Contact of a case | 8 |
| Accidental stick with a contaminated object | 7 |
| Medical employment | 4 |
| History of surgery | 4 |
| Application of a tattoo | 2 |
| Dialysis contact | 2 |
| Blood transfusion | 1 |
*Cases may have multiple risk factors
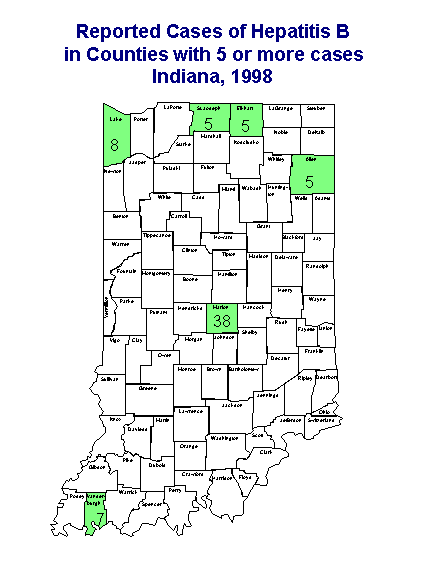
Figure 3.
Hepatitis C
Cases
= 4Crude rate
(per 100,000 population) = 0.07
In 1998, Indiana reported four persons residing in three counties who tested positive for the hepatitis C antibody and met the case definition for acute hepatitis C infection. Acute hepatitis C is reportable in Indiana. An acute case of hepatitis C must meet the following criteria: 1) discrete onset of illness, 2) jaundice or serum aminotransferase levels greater than 2.5 times the upper normal limit, 3) test negative for hepatitis A and hepatitis B, and 4) antibody to hepatitis (anti-HCV) verified by a supplemental test. Two people were hospitalized, three reported jaundice, and there was one death reported. Two cases were female. All were white. Risk factors for acquiring hepatitis C identified by the cases are shown below.
Table 2.
Frequency of Patient-Reported Risk Factors
Hepatitis C, Indiana 1998
Risk Factor |
Number
of |
| Transfusion | 1 |
| Injectable drugs | 1 |
| Dialysis | 1 |
| Medical Employment | 1 |
| Dental work in past 6 months | 1 |
*
Multiple positive responses possible
Cases in Indiana prior to 1997 were reported "Hepatitis C and NonA/NonB
Hepatitis" combined. In 1997, the present case definition for hepatitis C became
effective and hepatitis C is now reported separately. The case rate for hepatitis C in
1997 was 0.1/100,000 population.
Presently, the Indiana Communicable Disease Reporting Rule for Physicians, Hospitals, and Laboratories does not require the reporting of antibody to hepatitis C or any other test for hepatitis C. The tests available include the Enzyme Immunoassay (EIA), usually the first test ordered, the Recombinant Immunoblot Assay (RIBA), a supplemental test to confirm a positive EIA, and several tests to detect hepatitis C RNA virus. Physicians, hospitals, and laboratories have been voluntarily reporting laboratory tests positive for hepatitis C. There were 4,062 tests reported, representing 3,794 persons living in 88 counties. Sixty-two percent (62%) of the reported tests were performed on males. Table 3 illustrates the percentage of persons tested for hepatitis C by the above listed tests:
Table 3.
Percentage of persons tested for hepatitis C
by EIA, RIBA, and/or RNA testing, 1998*
Percent of |
EIA |
RIBA |
RNA |
1% |
X |
X |
X |
14% |
X |
X |
|
8% |
X |
X |
|
68% |
X |
||
0.2% |
X |
X |
|
2% |
X |
||
7% |
X |
*Voluntarily reported to ISDH