STIs: Antibiotic Resistant Gonorrhea
Overview
Gonorrhea is the second-highest reportable condition in the nation, behind Chlamydia. With the volume of cases our state sees each year, it is important to ensure we can collect as much information about the patient and their healthcare experience as possible through enhanced surveillance. This includes information on whether the patient was treated or not, what signs/symptoms of the disease they experienced, and any information about the frequency and types of sexual behaviors they practice. This helps inform interventions by public health programs to reduce the risk of STIs in the future. The Indiana Department of Health's Division of HIV/STI/Viral Hepatitis conducts enhanced surveillance on cases of gonorrhea through two, CDC-funded grants: Strengthening the U.S. Response to Resistant Gonorrhea (SURRG) and STD Surveillance Network (SSuN).
Below are resources for healthcare providers, local health departments, and the general public related to gonorrhea and the services our programs provide. If you have any questions related to the materials, contact the epidemiologist coordinator.
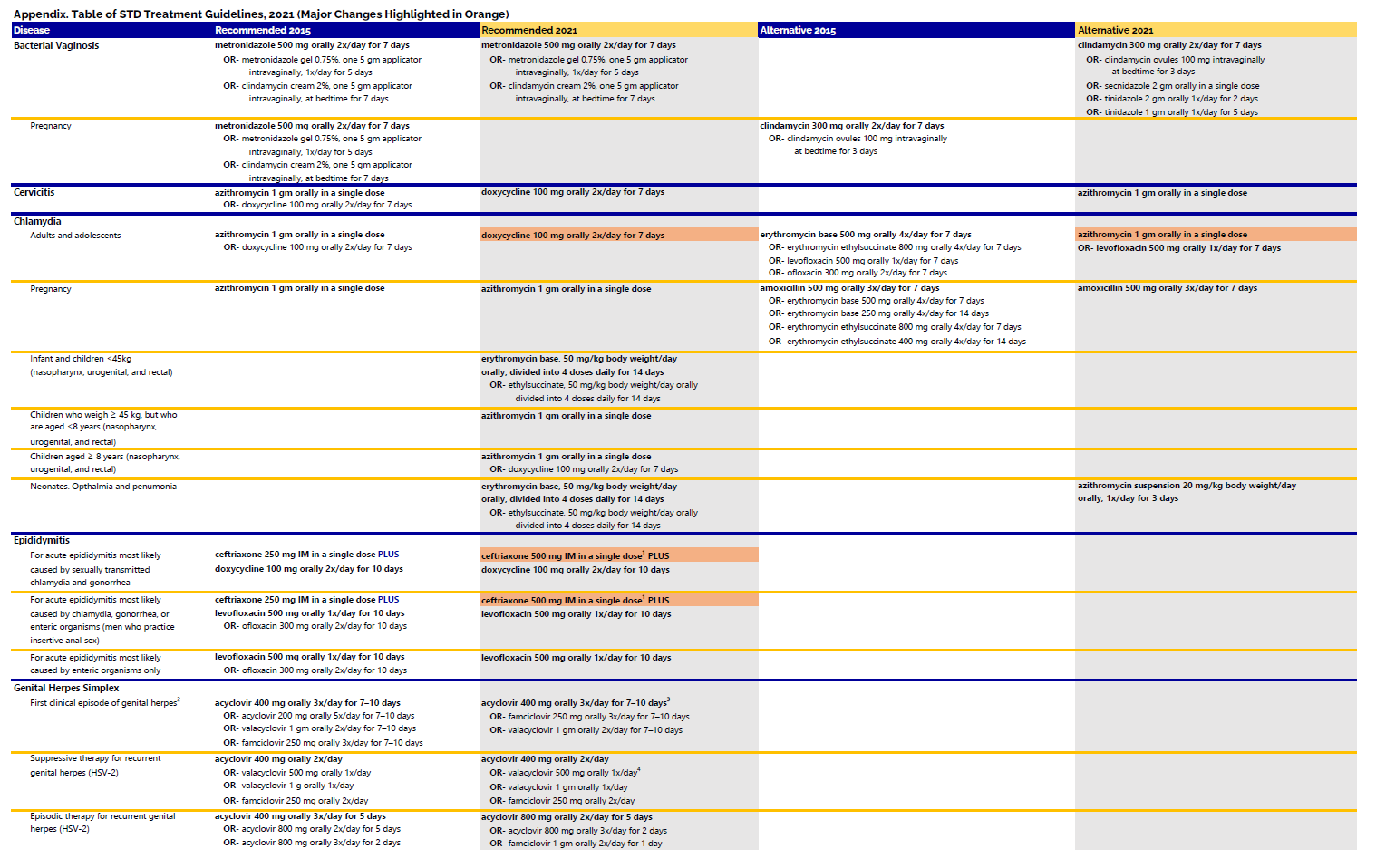
Gonorrhea is skilled at outsmarting the antibiotics that are used to kill it. For this reason, we must continuously monitor for antibiotic resistance and encourage the research and development of new drugs for gonorrhea treatment. In the CDC's 2020 STD Surveillance Report, updated trend figures (including 2011-2020, link below) highlighted not only the increasing concern of azithromycin resistance among gonococcal isolates, but also the impact of the COVID-19 pandemic on STI numbers.
Increases in STIs, especially for gonorrhea, continue in Indiana. Below is a link to an interactive map showing the rates of gonorrhea cases per county for the state in 2019. County rates are compared to those of the national average reported in the 2019 STD Surveillance Report. Counties with case counts of less than 20 have an asterisk by their rate (*) and those with counts less than five have their rate suppressed.
In order to provide resources for local health departments to prepare and respond to outbreaks of antibiotic-resistant gonorrhea (ARGC), Indiana has partnered with the CDC to create materials that can be used to create press releases or other formal communications during an outbreak.
Communication Materials
There is also a compilation of resources on the CDC's website specifically tailored for ARGC outbreak preparedness. This includes a toolkit for hosting an interactive outbreak simulation tabletop exercise and a template for creating an outbreak response plan for STIs in general, as well as for just ARGC. The site also includes an informative syphilis outbreak detection guide to help local jurisdictions determine if and when an outbreak may be occurring in their area. Use the link below to visit the STI Outbreak Response Resources on CDC's website.
Surveillance for resistant gonorrhea in the United States is conducted through several projects: the Gonococcal Isolate Surveillance Project (GISP), the enhanced Gonococcal Isolate Surveillance Project (eGISP), and Strengthening the United States Response to Resistant Gonorrhea (SURRG). Antibiotic susceptibility testing is an activity common to each project. Clinicians are asked to report any gonorrhea specimen with decreased cephalosporin susceptibility and any gonorrhea cephalosporin treatment failure to their state public health agency. All reports can be made to the SURRG epidemiologist coordinator.
If you suspect a patient may be a treatment failure and cannot perform gonorrhea culture for susceptibility testing at your agency, our team can coordinate the scheduling of culture testing and any necessary treatment through our partner site, the Bell Flower STD Clinic in Indianapolis, IN. If the patient is able to commute to this clinic, fill out the survey below to initiate the schedule coordination.
The following criteria should be considered while evaluating someone for a potential gonorrhea treatment failure:
- Patient had laboratory-confirmed Neisseria gonorrhoeae infection
- Patient received CDC-recommended treatment
- Patient subsequently had a positive N. gonorrhoeae test result (positive culture ≥72 hours or positive nucleic acid amplification test (NAAT) ≥7 days after treatment)
- Patient did not engage in any sexual activity in the 3-5 days following treatment
-OR- - Antimicrobial susceptibility testing (AST) of pre- or post-treatment isolate demonstrates ceftriaxone MIC ≥ 0.5 µg/mL
If you suspect a patient may be a treatment failure and your patient is unable to commute to the clinic, we have culture testing supplies that can be ordered by healthcare providers as needed at no-cost to the patient. The testing supplies include transport-media culture swabs used by the SURRG program to transport overnight specimens collected outside of the STI clinic to our partner lab, the Marion County Public Health Department Lab, for isolation and antibiotic susceptibility testing (AST). The supplies will also include a self-addressed stamped express shipping envelope as it is very important that the specimen makes it to the lab within 24 hours for testing. If you would like to order supplies for culture specimen collection at your agency, use the link below to complete a request form. All requests will be processed within 24 hours.
If your facility has already performed a gonorrhea culture on a patient and you would like to obtain AST from the SURRG lab, use the link below to request a shipping label to send your frozen sample to our lab for gonorrhea AST.
Request for GC Culture Testing Supplies and SURRG ASTOnce a person is treated for gonorrhea, they should return for testing in three months, especially if they are pregnant, females under the age of 25, or men who have sex with other men. People living with HIV should be screened for gonorrhea annually; those taking preventative treatment for HIV (Pre-Exposure Prophylaxis, PrEP) should be screened every 3-6 months, depending on when they are scheduled for re-evaluation. To assist providers in continuing best practices, below are resources for healthcare providers on treating patients and taking a sexual history, as well as educating patients about gonorrhea and antibiotic resistance.
Patient Education
Treatment
Provider Education
For more information regarding the updates to the gonorrhea treatment guidelines, check out the recorded webinar below from Healthcare Education and Training (HCET) which features guest speakers from the Centers for Disease Control and Prevention, the Indiana Department of Health, and the Marion County Public Health Department.
History of Resistance
Antibiotic resistance is bacteria’s ability to resist the effects of the drugs used to treat them. Bacteria that become resistant are no longer able to be killed by previously used drugs. Gonorrhea has developed resistance to nearly all of the antibiotics used for its treatment. We are currently down to one last recommended and effective class of antibiotics, cephalosporins, to treat this common infection. This is an urgent public health threat because gonorrhea control in the United States largely relies on our ability to successfully treat the infection.
Gonorrhea is skilled at outsmarting the antibiotics that are used to kill it. For this reason, we must continuously monitor for antibiotic resistance and encourage the research and development of new drugs for gonorrhea treatment.
SURRG in Indiana
In order to expand the antimicrobial resistance surveillance conducted by GISP, the CDC created the Strengthening the U.S. Response to Resistant Gonorrhea (SURRG) project in 2016. Similar to GISP, funded jurisdictions across the United States collect gonorrhea isolates that are tested for antimicrobial susceptibility. One of the new objectives for SURRG is to collect gonorrhea isolates from women and extra-genital infections, which were previously missed in GISP. In 2016, Indiana was chosen as one of eight sites to participate in the SURRG project.
Project Aims
The Indiana SURRG project aims to:
- Strengthen community coordination for the identification of resistant gonorrhea through increasing culture collection and antibiotic susceptibility testing (AST) via partnerships with clinical sites and the public health laboratory;
- Enhance timely surveillance and communication between government agencies, community organizations and medical facilities for rapid detection of resistant gonorrhea strains;
- Conduct in-depth disease intervention specialists (DIS) interviews with patients identified as having gonorrhea with increased resistance to develop social and sexual networks for analysis; enhance DIS case investigations to identify the transmission dynamics of resistant gonorrhea within local networks and create innovative intervention activities; and
- Increase community engagement and participation in gonorrhea prevention and control with outreach disease intervention related to social network analyses.
Partnerships
In partnership with the Marion County Public Health Department in Indianapolis, IN, Neisseria gonorrhoeae isolates are collected from two testing sites:
- Bell Flower STD Clinic (Marion County Public Health Department)
- Eskenazi Hospital Emergency Department
Screening tests for gonorrhea are available at all the above locations. If you test positive, a DIS may contact you to come in for treatment and a SURRG culture sample could be collected at that time. Opting into this test will allow a more comprehensive look at a patient’s infection and ensure the appropriate medication is being used to clear the infection. Speaking with the DIS will also help us understand how resistant gonorrhea is spreading through sexual networks and could aid public health workers in stopping disease transmission in the future.