Resources for Medical Providers
Clinicians
- The Opioid Epidemic and Smoking
- CDC Guidelines for Prescribers: Chronic Pain
- Indiana RX Guidelines Comparison of CDC vs. Indiana Prescribing Guidelines
- CDC Opioid Prescribing App for Prescribers
- Pharmacists and naloxone: Let's talk about it! - IDOH
- IDOH Brochure: Overdose Prevention Collaboration Programs
First Responders
- First Responder Precautions for Unknown Opioids - IDOH
- First Responder Compassion Fatigue - IDOH
- Naloxone Reporting Requirements for First Responders - IDOH
- First Responder Self-Care - IDOH
- Officer Safety Alert- Carfentanil
- Fentanyl Safety Recommendations
- Narcan Quick Start Guide
- Naloxone Atomizer Instructions
Other Resources
- INSPECT: Indiana's Prescription Drug Monitoring Program
- ISMA Online App
- NIDAMED
- SAMHSA Opioid Overdose Toolkit for Providers
- Xylazine for Providers - IDOH
- Emerging Drug Notification: Xylazine
- Emerging Drug Notification: N-pyrrolidino etonitazene
- Emerging Drug Notification: Bromazolam
- Emerging Drug Notification: Medetomidine
- Opioid Prescribing Guidelines
- Continuing Education (CE) Courses
- Medication-Assisted Treatment (MAT)
- Toxicology Surveillance
- Rapid Surveillance Reporting
In 2017, more than 6 million opioid prescriptions were dispensed to Indiana residents. Improving the way opioids are prescribed through clinical practice guidelines can ensure patients have access to safer, more effective pain treatment, while reducing the number of people who misuse, abuse or overdose from these drugs. The following guidelines have been developed and published by experts in the field of pain management to guide clinicians on best practices when it comes to prescribing opioids. Each guideline is tailored to a specified clinical setting.
Emergency Department
Indiana Guidelines for Opioid Prescribing in the Emergency Department
These guidelines provide emergency departments in Indiana a general approach to prescribing opioids for acute conditions. Included with the guidelines is a “Facility Action Checklist” that enables hospitals to review and compare their current practices and align them with the recommended guidelines.
Chronic Pain
Indiana Pain Management Prescribing Final Rule
In 2014, the Indiana Medical Licensing Board adopted a final rule that regulates physicians engaged in the practice of pain management prescribing, pursuant to Indiana Administrative Code 844 IAC 5-6. These regulations address the main factors of safe and effective prescribing practices that include: patient assessment, non-opioid treatment options, patient information consent, patient follow-ups, INSPECT reports, drug monitoring tests, a daily high dose threshold and a treatment agreement.
CDC Guidelines for Prescribing Opioids for Chronic Pain
The CDC’s guidelines for prescribing opioids intends to increase communication between providers and patients about the risks of opioid treatment for chronic pain, improve the safety and effectiveness of long-term opioid treatment and re-emphasize follow-ups to evaluate benefits and harms of continued therapy. These guidelines apply to primary care providers treating adults with chronic pain for more than three months, excluding cancer, palliative and end-of-life care. For a more detailed description of CDC’s guidelines, click here.
CDC Videos
The CDC has created an interactive online training series aimed to help healthcare providers apply CDC’s recommendations in clinical settings through patient scenarios, videos, knowledge checks, tips and resources. Providers can gain a better understanding of the recommendations, the risks and benefits of prescription opioids, non-opioid treatment options, patient communication and risk mitigation. Each standalone module is self-paced and offers free continuing education credit (CME, CNE, and CEU). Visit the CDC website for more information.
CDC Mobile App
The CDC has created an opioid guideline mobile app designed to help providers apply the recommendations of CDC’s Guideline for Prescribing Opioids for Chronic Pain into clinical practice by putting the entire guideline, tools and resources in the palm of their hand. The application includes a morphine milligram equivalent calculator, summaries of key recommendations, a link to the full guideline and an interactive motivational interviewing feature to help providers practice effective communications skills and prescribe with confidence. Visit the CDC website for more information.
Additional Resources:
- CDC's Guideline Resources: Clinical Tools
- Jama Special Communication regarding CDC Guidelines for Prescribing Opioids for Chronic Pain
Comparison of CDC Guidelines to Indiana Prescribing Rule
The variety of guidelines published by various institutions can often be difficult to compare and contrast. In response to this, the Indiana State Medical Association has compiled a document that compares CDC’s Guidelines for Prescribing Opioids for Chronic Pain with Indiana’s Pain Management Prescribing Final Rule. Although both sets of guidelines are aimed at improving the safety and effectiveness of opioid prescribing practices, the Indiana requirements are tailored more to the state of Indiana, while the CDC’s recommendations apply nationally. Physicians in Indiana may still apply the CDC’s recommendations in their opioid prescribing practices, even if those guidelines are not addressed in Indiana’s requirements.
Acute Pain
The Indiana Guidelines for the Management of Acute Pain
The Indiana Guidelines for the Management of Acute Pain guidelines address safe, appropriate and effective opioid prescribing practices for outpatient management of acute pain. They may be applied to patients of all ages presenting acute pain, but they may not apply to acute pain resulting from a chronic condition.
Additional Resources
Co-prescribing Naloxone to Patients at Risk of Overdose
Co-prescribing naloxone is encouraged by a broad range of stakeholders to help reverse the effects of an opioid overdose for high-risk patients. This resource provided by the American Medical Association (AMA) describes how to determine when it is clinically appropriate to co-prescribe naloxone and provides additional considerations, such as how to approach a patient you wish to co-prescribe naloxone to.
In order to participate in the continuing education program, you will need to create an IN-TRAIN account if you do not already have one.
Currently, there are no Continuing Education Courses offered by the IDOH Oral Health Program; however, there are 4 Non-CE Courses provided by the Oral Health Program.
Continuing Education Courses on IN-TRAIN
- Measuring Health Disparities - Course ID 1087494
- Rural Health: Exploring The Growing Disparities - Course ID 1078208
What is medication-assisted treatment?
- Medication-assisted treatment (MAT) is the use of FDA-approved medications, along with appropriate counseling and behavioral therapy, to treat substance use disorder and sustain recovery.
Physician training and education on MAT
- For a physician to be eligible to prescribe or dispense buprenorphine for the purpose of MAT, he or she must qualify, apply for a physician waiver, and complete an eight-hour buprenorphine waiver training.
- Residential Substance Abuse Treatment (RSAT) Training Tool: Medication-assisted treatment (MAT) for offender populations provides evidence-based information on the application and effectiveness of MAT among the offender population during incarceration and as they are released and re-enter the community.
- The Addiction Technology Transfer center (ATTC) provides online training designed to enhance professionals’ knowledge and skills related to MAT and increasing the use of MAT.
- Substance Abuse and Mental Health Services Administration’s (SAMHSA) Medication-Assisted Treatment of Opioid Use Disorder Pocket Guide provides physicians with information on approved medications, screening and assessment tools, and best practices for patient care.
- Webinars and educational videos provided by Providers Clinical Support System.
Visit the SAMHSA website or call SAMHSA at 1-866-BUP-CSAT (1-866-287-2728) for more information about physician waiver qualifications, treatment training in your area or to obtain a waiver.
Opioid ECHO Project
- The Opioid ECHO project is a free partnership between local primary care providers and a team of specialists from Indiana University (IU). It aims to improve the treatment of opioid use disorder in rural and underserved areas by educating primary care clinicians through virtual video-conferencing to provide specialty care services.
- IU will conduct three separate ECHO clinics for the following disciplines: prescribers, behavioral health specialists, and community health workers/peer recovery workers.
- Each clinic has a curriculum that is 12 sessions long. After the curriculum is completed, the clinic will restart at the beginning with a new cohort of participants.
- Visit the Opioid ECHO Project website if you are interested in joining.
Additional MAT Resources
- Physicians must confirm that that they will not prescribe buprenorphine to more than 100 patients during the first year of obtaining their buprenorphine waiver. Following the first year, physicians can apply for approval to treat up to 275 patients. Learn more on applying to increase patient limits after the first year.
- Unlike physicians, pharmacists do not need a buprenorphine waiver to dispense buprenorphine. No additional credentials are required for a pharmacist to dispense a schedule III substance, such as buprenorphine. However, when the medication has been prescribed they are required to go to the Buprenorphine Pharmacy Lookup and verify the prescribing physician’s buprenorphine certification. Learn more about verifying physician waivers for pharmacists.
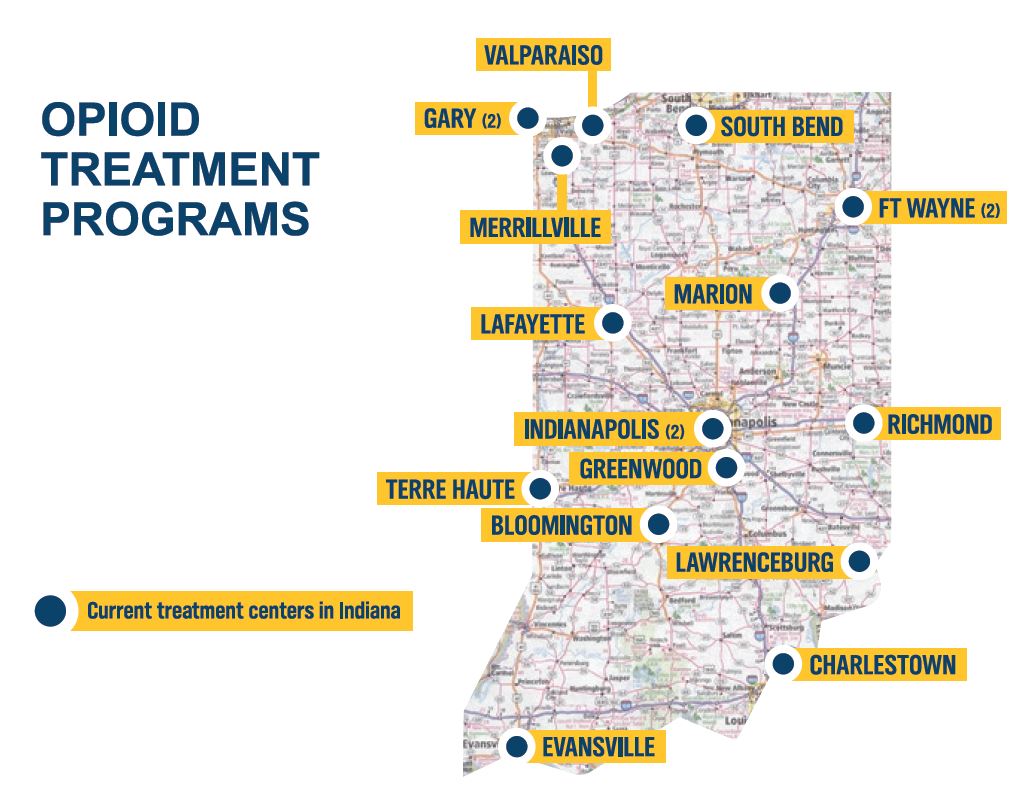
- As of 2020, there are 18 opioid treatment programs (OTPs) in the state of Indiana (demonstrated by the dark blue pins on the map). To find an OTP, visit https://www.in.gov/recovery/.
Between 1999 and 2020, drug overdose deaths increased by 319 percent in Indiana. The recent surge in the supply of illicit fentanyl is contributing to the rapid rise of drug overdose deaths. This highlights the need for timely and accurate data to monitor changes in the types of opioids involved in overdose deaths.
The Indiana Department of Health’s (IDOH’s) ability to respond quickly and appropriately to the drug overdose epidemic depends on complete and timely data. Historically, drug overdose deaths have too often been classified on death certificates as resulting from “multi-drug toxicity” or simply “drug overdose” without specifying the drug that caused the fatal overdose. Comprehensive toxicology testing is essential to properly identify which drugs, specifically opioids, are associated with overdose deaths.
IDOH is improving fatal drug overdose reporting by funding standardized toxicology testing on suspected drug overdose deaths. Under Indiana Code 36-2-14-6 (b), all Indiana coroners are required to conduct toxicology screenings to gather information on suspected controlled substances in fatal overdose cases.
Coroners must take the following steps if they reasonably suspect the cause of a person’s death to be an overdose of a controlled substance, whether it was accidental or intentional:
1. Obtain any relevant information about the decedent maintained from the INSPECT program;
2. Extract and test certain bodily fluids of the decedent;
3. Report test results to IDOH; and
4. Provide the department notice of the decedent's death, including any information related to the controlled substances involved, if any.
This legislation has enhanced the state's ability to respond quickly and appropriately to control the drug overdose epidemic by providing more rapid, consistent and extensive data.
As part of this new law, coroners will also be required to create an account to access the INSPECT records of a person who has died of a suspected drug overdose. An INSPECT report summarizes the controlled substances a patient has been prescribed, the practitioner who prescribed them, and the dispensing pharmacy where the patient obtained them. To read more about INSPECT, visit the website and scroll down to the section on Law Enforcement utilizing INSPECT to review the policies regarding coroners and law enforcement.
If you have questions about the toxicology program, please email indianatrauma@health.in.gov or contact the Trauma and Injury Prevention Division director, Brian Busching, at BBusching@health.in.gov or 317-234-2865.
Toxicology Reports
The reports below have been created by partners at the Wayne State University Center for Behavioral Health and Justice.
2019 Multi-Drug Toxicology Report
2020 Multi-Drug Toxicology Report
Overview
To more rapidly detect overdose outbreaks or sharp increases in overdose deaths, IDOH will be partnering with coroners to collect preliminary data on suspected overdose deaths (i.e., deaths suspected to involve substances before receipt of forensic toxicology data). Awarded coroners have been asked to provide information on the decedent within a month of the date of death. The data requested includes background on the drug overdose scene and minimal identifiable information on the decedent. IDOH will collect this information using the Coroner Case Management System (CCMS), a repository in which coroners can manage their cases.
Suspected Drug Overdose Case Definition
A fatal drug overdose is confirmed by positive toxicology results and is certified by a coroner/medical examiner as such. In order to quickly identify possible overdoses before confirmed toxicology results, we ask that coroners report cases if they meet the following case definition:
- Drug paraphernalia suggestive of use was found at/around the scene OR
- Clinical evidence suggestive of an opioid overdose including pulmonary/cerebral edema, bladder distension, pinpoint pupils, injections sites, foam cone, etc. OR
- Unexpected death with a diagnosis of substance use disorder OR
- Evidence of stimulant overdose including seizures and multiple organ failures resulting from heatstroke OR
- Any other evidence indicative of a drug overdose/prior overdose.
Within CCMS, IDOH will be using the following variables to track suspected overdose deaths:
- Case Criteria Additional Information: Potential Drug Overdose or “PDO”
- Injury Date
- Decedent First Name
- Decedent Last Name
- Gender
- Race
- DOB
- Decent Zip Code/Postal Code
- Death Date
- Place of Death
- Scene Description
- Injury Place
- Describe How Injury Occurred/Injury Occurred Description
Coroners must complete these fields in CCMS when filling out cases for each decedent. Instructions on completing this can be found here.
Death Investigation Guide
Click here for additional guidance on detecting a drug overdose on scene.