Botulism
Botulism (“BOT-choo-liz-um”) is a rare but serious illness caused by a toxin that attacks the body’s nerves and causes difficulty breathing, muscle paralysis, and even death. This toxin is made by Clostridium botulinum and sometimes Clostridium butyricum and Clostridium baratii bacteria. These bacteria can be spread by food and sometimes by other means.
- What is botulism?
The bacteria that make botulinum toxin are found naturally in many places, but it’s rare for them to make people sick. These bacteria make spores, which act like protective coatings. Spores help the bacteria survive in the environment, even in extreme conditions. The spores usually do not cause people to become sick, even when they are eaten. Under certain conditions, these spores can grow and make one of the deadliest known toxins. The conditions in which the spores can grow and make toxin are:
- Low-oxygen or no oxygen (anaerobic) environment
- Low acid
- Low sugar
- Low salt
- A certain temperature range
- A certain amount of water
For example, improperly home-canned, preserved, or fermented foods (i.e. sauerkraut or kombucha) can provide the right conditions for spores to grow and make botulinum toxin. When people eat these foods, they can become seriously ill, or even die, if they don’t get proper medical treatment quickly.
Link to CDC website: https://www.cdc.gov/botulism/index.html
- What are the different types of botulism?
Foodborne botulism
- Foodborne botulism can happen by eating foods that have been contaminated with botulinum toxin. Common sources of foodborne botulism are homemade foods that have been improperly canned, preserved, or fermented. Though uncommon, store-bought foods also can be contaminated with botulinum toxin.
Infant botulism
- Some infants get botulism when the spores get into their intestines, grow, and produce the toxin. The reason for this is unknown.
Wound botulism
- Wound botulism can happen if the spores get into a wound and make a toxin. People who inject drugs have a greater chance of getting wound botulism. People who have had a traumatic injury, such as a motorcycle crash, or surgery also can get wound botulism.
Iatrogenic botulism
- Iatrogenic botulism can happen if too much botulinum toxin is injected for cosmetic reasons, such as for wrinkles. It also can happen if too much of the toxin is injected for medical reasons, such as for migraine headaches.
Adult intestinal toxemia
- Adult intestinal toxemia is also known as adult intestinal colonization. This kind of botulism is very rare. It can happen if the spores of the bacteria get into an adult's intestines, grow, and produce the toxin (similar to infant botulism). Scientists do not know why people get this kind of botulism. People with serious health conditions affecting the gut might be more likely to get ill.
- What are the symptoms of botulism?
Botulism symptoms can vary based on the type of botulism. Below are the differences in symptoms.
Possible signs and symptoms for all individuals Possible signs and symptoms included with foodborne botulism Possible signs and symptoms included with infants - Difficulty swallowing
- Muscle weakness
- Double vision
- Drooping vision
- Blurry vision
- Slurred speech
- Difficulty breathing
- Difficulty moving the eyes
- Vomiting
- Nausea
- Stomach pain
- Diarrhea
- Constipation
- Poor feeding
- Drooping eyelids
- Pupils that are slow to react to light
- Face showing less expression than usual
- Weak cry that sounds different than usual
- Difficulty breathing
*People with botulism might not have all these symptoms at the same time.
The symptoms all result from muscle paralysis caused by the toxin. If untreated, the disease may progress and symptoms may worsen to cause full paralysis of some muscles, including those used in breathing and those in the arms, legs, and trunk (part of the body from the neck to the pelvis area, also called the torso).
- How can botulism be prevented?
If you or someone you know has symptoms of botulism, immediately see your doctor or go to the emergency room.
Foodborne botulism
In foodborne botulism, symptoms generally begin 18 to 36 hours after eating a contaminated food.
Many cases of foodborne botulism have happened after people consumed home-canned, preserved, or fermented foods that were contaminated with toxin. The foods might have become contaminated if they were not canned (processed) correctly. For commercially canned foods, a can that appears to be bulging, leaking, or damaged is a sign of possible contamination. Upon opening, the food may also be contaminated if there is spurting, foaming, or it appears moldy or smelly.
Foods with low acid content are the most common sources of home-canning related botulism cases. Examples of low-acid foods are:
- Asparagus
- Green beans
- Beets
- Corn
- Potatoes
New sources of foodborne botulism continue to be identified. Contamination can happen when food is handled improperly when it is made, when it is stored, or when it is used by consumers. Some examples of foods that have been contaminated are:
- Chopped garlic in oil
- Canned cheese sauce
- Canned tomatoes
- Carrot juice
- Baked potatoes wrapped in foil
In Alaska, most cases of foodborne botulism are caused by fermented fish and other aquatic animals.
If you preserve, can, or ferment your own foods, you can reduce the chance of these foods giving you, your family, or friends botulism by:
- Following safe home canning instructions as recommended by the U.S. Department of Agriculture in the USDA Complete Guide to Home Canning.
- Following all instructions for washing, cleaning, and sterilizing items used in canning
- Using pressure canners for low-acid foods like potatoes, most other vegetables, and meats
Everyone can reduce their chances of getting botulism by:
- Refrigerating homemade oils infused with garlic or herbs and throwing away any unused oils after four days
- Keeping potatoes that have been baked while wrapped in aluminum foil hot (at temperatures above 140°F) until they are served or refrigerating them with the foil loosened
- Refrigerating any canned or pickled foods after you open them
Infant botulism
Most infant botulism cases cannot be prevented because the bacteria that causes the disease is in soil and dust. This bacteria can be found inside homes on floors, carpet, and countertops —even after cleaning. For almost all children and adults who are healthy, ingesting botulism spores is not dangerous and will not cause botulism (it is the toxin that is dangerous). For reasons scientists do not understand, some infants get botulism when the spores get into their digestive tracts, grow, and produce the toxin.
Honey can contain the bacteria that causes infant botulism, so do not feed honey to children younger than 12 months. Honey is safe for people 1 year of age and older. Learn more about infant botulism from the Infant Botulism Treatment and Prevention Program.
Prevent wound botulism by keeping wounds clean. If wounds appear infected, seek medical care quickly. A wound might be infected if it is:
- Red
- Swollen
- Painful
- Warm to the touch
- Full of pus or other drainage
- Accompanied by fever
Not all wounds with botulism show these general symptoms of a wound infection. If you have a wound and begin to have symptoms of botulism, seek medical care immediately.
People who inject drugs are more likely to get wound botulism than people who do not inject drugs. People who get botulism from injecting drugs might not have an obviously infected injection site. Learn more about preventing wound botulism caused by injecting drugs.
Wound botulism may happen after traumatic injuries, such as motorcycle crashes, and surgeries. Be alert to signs of infection.
Adult intestinal colonization
Adult intestinal colonization (also called adult intestinal toxemia) is a very rare type of botulism. People who have health conditions that change the structure or proper workings of their intestines (gut) may be at higher risk. Only a handful of people have been diagnosed with adult intestinal toxemia, and scientists do not fully understand how a person gets this type of botulism. It may be similar to infant botulism, which cannot be prevented.
Iatrogenic botulism
You can prevent iatrogenic (an illness caused by medical examination or treatment) botulism by getting injections of botulinum toxin only by licensed practitioners. If you need an injection of botulinum toxin for a medical condition, your doctor will choose the safest dose. If you get an injection of botulinum toxin for cosmetic reasons, be sure to go to a licensed professional.
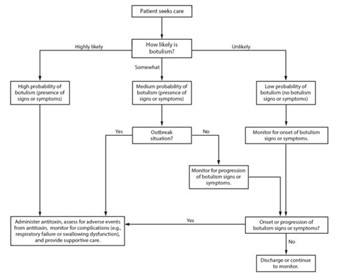
Information for Health Care Providers
If there is a suspected case of botulism, each scenario may be different. Suspect botulism responses should be initiated immediately to avoid serious outcomes, including death. Do not wait for laboratory confirmation to initiate consultation or treatment. Shown below are the general rules for Indiana, as well as who to contact and next steps, depending on what is needed.
- Botulism is immediately reportable upon suspicion per 410 IAC 1-2.5-75 & 76
- First reports SHOULD NOT be through NBS
- Please call to report!
- We cannot do rule-out testing for botulism. If we’re testing, we’re treating.
- Decision to treat is based on clinical presentation and is made by the physician, along with IDOH and CDC
- IDOH and CDC need to authorize release of antitoxin should a patient truly need it.
- Botulism Contacts
- Medical Decision Making
- Testing for Botulism
- Sample Collection
- Shipping to IDOH Lab
| Contact Type | Name | Phone Number |
|---|---|---|
| Indiana Department of Health |
Enteric Epidemiologist Epi on Call (After Hours) |
(317) 233-7125 (317) 233-1325 |
| Non-Infant Patients | CDC Emergency Operations Center | (770) 488-7100 |
| Infant Patients | California Department of Health Infant Botulism Treatment and Prevention Program | (510) 231-7600 |
Botulism Treatment Logistics
Botulism Anti-Toxin (Non-Infant Patients)
Once consultation and approval are appropriately discussed between CDC, IDOH, and the provider, CDC will release the antitoxin from the nearest Quarantine Station (Chicago). CDC will then help coordinate with the given facility and the quarantine station for proper shipment and administration.
CDC produced a video to guide healthcare professionals through preparing and administering antitoxin to treat botulism. The video also answers some questions often asked during clinical consultations for suspect botulism cases. As a companion to the antitoxin’s FDA package insert, the video can prepare patient care teams to act when the antitoxin arrives. If a large botulism outbreak occurs, public health officials and first responders may also turn to the video for guidance.
Once consultation and agreement for the release of BabyBIG is agreed between California Department of Health and the calling provider, California will organize the transfer of BabyBIG to the given facility.
Testing for botulism is the same for any type of botulism. To have botulism tested, botulism anti-toxin or BabyBIG are required to be given. In Indiana, testing is performed in Ohio, however samples are still required to be sent to the Indiana Department of Health Laboratory. The Ohio tests include mouse bioassay, polymerase chain reaction (PCR), and culture. Testing can vary in time from nine days for a negative result and 17 days for a positive result. IDOH personnel will update all parties involved as soon as possible when results are shared from Ohio.
To collect specimens, it is best practice to have it collected prior to administration of anti-toxin/BabyBIG. Traditionally, botulism is tested in stool specimens or blood, but if other specimens are requested, please consult with the Indiana Department of Health. See attached for more specific requirements for testing. For samples to be thoroughly processed, without delay, please also fill out the Microbiology Specimen Submission Form from Ohio.
Samples are to be sent initially to the Indiana Department of Health Laboratory, addressed to the Biological Preparedness division (see below for shipping specifics). For specimens sent to IDOHL, please also submit it in LimsNET.
ATTN: Biothreat Laboratory
550 W. 16th St, Suite B
Indianapolis, IN 46202
CLIA 15D0662599
Samples are to be sent initially to the Indiana Department of Health Laboratory, addressed to the Biological Preparedness division (see below for shipping specifics). For specimens sent to IDOHL, please also submit it in LimsNET.
ATTN: Biothreat Laboratory
550 W. 16th St, Suite B
Indianapolis, IN 46202
CLIA 15D0662599
Page last updated/reviewed: November 2024
